Information
Journal Policies
The Role of Oxidative Stress in Children with Acute and Chronic Immune Thrombocytopenic Purpura
Erol Erduran1*,Semiha Cakmak2,Gokce Pinar Reis3,Yuksel Aliyazicioglu4,Aysenur Bahadir5,Selim Demir6
2.Assistance Professor in Pediatrics. Recep Tayyip Erdogan University, Rize-Turkey.
3,5.Department of Pediatric Hematology and Oncology, Faculty of Medicine, Pediatrics, Karadeniz Technical University, Trabzon-Turkey.
4.Professor in Biochemistry, Karadeniz Technical University Faculty of Medicine, Department of Medical Biochemistry, Trabzon- Turkey.
6.Assistant Professor. Karadeniz Technical University, Faculty of Health Sciences, Department of Nutrition and Dietetics, 61080, Trabzon-Turkey.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The etiopathogenesis of autoimmune diseases is attributable to oxidative injury. This study therefore investigated whether or not oxidative injury is involved in the etiopathogenesis of acute and chronic ITP in childhood.A product of DNA oxidation - 8-OH-deoxyguanosine (8-OHdG), a product of protein oxidation - protein carbonyl, and a product of lipid peroxidation - malondialdehyde (MDA), and total oxidative stress (TOS) and total antioxidant capacity (TAC) were investigated in 27 patients with chronic and acute ITP and 31 controls.
A statistically significant difference was determined in 8-OHdG, MDA, TAC and TOS levels between the three groups (p=0.000). 8-OHdG levels in acute ITP were statistically higher compared to those in chronic ITP and in the controls (p=0.013 and p=0.000, respectively). MDA levels in chronic and acute ITP were statistically significantly higher compared to the controls (p=0.000). TAC levels in chronic ITP were statistically significantly higher than in the controls (p=0.004). TOS levels in acute and chronic ITP were significantly higher compared to those in the controls (p=0.000).
8-OHdG, MDA, TAC and TOS were higher in acute and chronic ITP patients. We think that oxidative damage is involved in the pathogenesis of acute and chronic ITP.
Oxidation, idiopathic thrombocytopenic purpura, 8-hydroxydeoxyguanosin, malondialdehide, protein carbonyl,Hematology
1. Introduction
Primary immune thrombocytopenia, previously referred to as idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired disorder characterized by mucocutaneous bleeding and isolated thrombocytopenia below 100,000/uL in the absence of any specific cause of the thrombocytopenia [1]. It is further classified according to its duration since diagnosis as follows; newly diagnosed (< 3 months), persistent (3-12 months) and chronic (>12 months) [2]. Platelets coated with antibodies are phagocytized by macrophages homing in the reticuloendothelial system (RES) [3]. Anti-platelet antibodies are responsible for the pathogenesis in 50-80% of children with acute ITP. Various mechanisms have been implicated in the pathogenesis of acute ITP because anti-platelet antibodies have not been detected in 20% of patients with acute ITP [4,5].
Oxidative stress is caused by an imbalance between the production of reactive oxygen and biological system’s inability detoxify the reactive intermediates. Oxidative stress is responsible for the pathogenesis of many autoimmune diseases. Reactive oxygen substances (ROS) are important signal molecules for immune system cells [6]. ROS inhibit the activation of T lymphocytes and cause the development of autoimmune diseases. It has been suggested that oxidative injury may play a role in the pathogenesis of ITP since this is also an autoimmune disease [7,8]. Lipopolysaccharides, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) activate intracellular NADPH oxidase, and oxygen consumption increases as a result. Superoxide anion radical (O2-) is synthesized, and O2- is converted to hydrogen peroxide (H2O2). H2O2 mediates hydroxyl radical (OH-) synthesis via the Fenton reaction in the presence of iron. OH-causes lipid, protein and DNA damage. Nitrite oxide synthesized from arginine is converted to reactive nitrogen molecules, such as peroxynitrite (ONOO-), by increased intracellular O2-. ONOO-reduces intracellular –SH groups. Reduced -SH groups lead to the oxidation of glutathione, and an increased oxidized / reduced glutathione ratio results in inflammatory cytokine transcription, such as TNF-α, inducible nitric acid synthase, IL-1β, cytosolic phospholipase A2 and cyclooxygenase-2, via the inhibitor kappa kinase [7]. Persistent oxidative stress causes lipid peroxidation, and reactive aldehydes are formed, evidenced by elevated MDA levels in the plasma of ITP patients. Lipid peroxidation and oxidative protein modification are also observed in patients with ITP as a result of oxidative damage [8].
Oxidation products or defense mechanisms against oxidation, such as total oxidative stress (TOS), total anti-oxidant capacity (TAC) and the oxidative stress index (OSI), have been used to determine oxidative stress. Oxidation products include 8-hydroxydeoxyguanosine (8-OHdG) and 5-hydroxyurasil for DNA, protein carbonyl for proteins, and thiobarbituric acid reactive products (TBAR-RS), malondialdehyde (MDA) and F2 isoprostanes for lipids [9].
This study investigated levels of serum 8-OHdG, MDA and protein carbonyl, together with TOS and TAC, in acute and chronic ITP in order to emphasize the role of oxidative stress in the pathogenesis of ITP.
2. Materials And Methods
Twenty-seven patients (17 girls, 10 boys) aged 5-14 with chronic ITP, 27 patients (15 girls, 12 boys) aged 0.4- 9.9 with acute ITP and 31 healthy children (18 girls, 13 boys) aged 3 – 12 representing the control group were enrolled in the study.
Inclusion criteria for chronic ITP patients were [10,11]:
1. Platelet counts lower than 100,000/uL over 12 months following the diagnosis of acute ITP.
2. Patients without congenital or acquired bone marrow failure, Kasabach-Merritt Syndrome, hemolytic uremic syndrome, Wiskott-Aldrich syndrome, type 2b von Willebrand disease, systemic lupus erythematosus (SLE), anti-phospholipid antibody syndrome (APAS), autoimmune lymphoproliferative syndrome (ALPS), leukemia, solid tumors, immune deficiency, disseminated intravascular coagulation, septicemia, organic acidemias, or cobalamin or folic acid deficiency in association with platelet counts < 100,000/uL over 12 months.
Inclusion criteria for acute ITP patients were [10]:
Thrombocytopenia (< 100,000/uL) associated with
1. A history of viral infection in the previous 2-3 weeks
2. Absence of acute β hemolytic streptococcus, Parvovirus, viral hepatitis A, B or C viruses, human immune deficiency virus, Ebstein Barr virus, cytomegalovirus, varicella zoster virus, rubella, measles, mumps, toxoplasmosis, malaria, kala-azar infections or any infection that causes thrombocytopenia
3. Absence of ‘wrong blood’ transfusion history
4. Absence of history of use of any medication that causes thrombocytopenia
5. Absence of autoimmune diseases such as SLE, APAS or ALPS that cause thrombocytopenia
6. Absence of lymphoreticular/lymphoid system malignancies
7. Absence of any history of familial thrombocytopenia
Local ethics committee was approved the study, informed consent had been given by parents. Blood samples were collected at diagnosis from patients with acute ITP and one month after interruption of treatment such as intravenous immunoglobulin, cyclosporine and immuno suppressive drugs in patients with chronic ITP. None of patients with chronic ITP received splenectomy prior to the start of our study.
Complete blood count, Coombs’ direct antiglobulin test, anti-nuclear antibody, 8-OHdG, protein carbonyl, MDA, TOS, TAC and OSI were studied in the blood samples obtained from patients with acute and chronic ITP. OSI was calculated using the formula:
OSI= TOS (umol/L) / TAC (mmoltrolox equivalent/L) x 100 Serum immunoglobulin, serum cobalamin and folic acid, hepatitis C serology and H. pylori antigen in stool were investigated in patients with chronic ITP.
Complete blood count was studied using a Beckman Coulter LH 750 device. Blood samples were placed into gel-containing tubes for TOS and TAC analyses, and into tubes containing EDTA for MDA, 8-OHdG, and protein carbonyl analyses. Blood samples were centrifuged at 3500 rpm for 10 minutes and stored at -80 0C in a deep freeze until analysis. TAC (cat. no: RL017, Mega Tıp Sanayii ve Ticaret Ltd., Istanbul-Turkey) and TOS (cat. no: RL019, Mega Tıp Sanayii ve Ticaret Ltd., Istanbul-Turkey) were studied using the spectrophotometric method [11,12]. MDA was studied using the TBAR-RS method [11]. 8-OHdG was determined using a specific kit (cat. no: 049KOG-HSE10/E, JalCA, Tokyo-Japan) and protein carbonyl using high performance liquid chromatography [13,14].
3. Statistical Analyses
Statistical analyses were performed on SPSS 13 (Statistical Package for Social Sciences) for Windows software. ANOVA (Post hoc Bonferroni) was used to assess parametric variables and Kruskal Wallis analysis of variance was used for non-parametric variables. P < 0.05 was considered statistically significant. Data were calculated as arithmetic mean ± standard deviation (x± SD).
4. Results
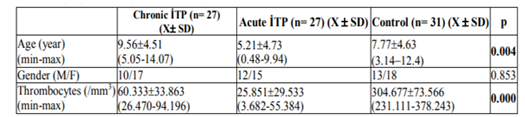
Mean ages were 9.56 ± 4.51 years in patients with chronic ITP, 5.21 ± 4.73 in patients with acute ITP and 7.77 ± 4.63 in the controls. The mean age of the patients with chronic ITP was statistically significantly higher compared to patients with acute ITP and the controls (p=0.003). No statistically significant difference was present between the mean ages of the patients with acute ITP and controls (p> 0.05). Mean platelet count was 60.333 ± 33.863/uL (26.470-94.196) in the patients with chronic ITP, 25.851 ± 29.533/uL (3.682-55.384) in patients with acute ITP and 304.677 ± 73.566/uL (231.111-378.243) in the controls (p=0.000). Age, gender and platelet counts of acute and chronic ITP and controls are shown in Table 1.
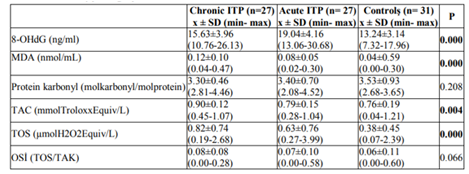
The mean serum 8-OHdG, MDA, protein carbonyl, TAC, TOS and OSI values for all three groups are shown in Table2. Post-hoc pair wise comparison analysis was performed between the data identified as exhibiting statistically significant differences.
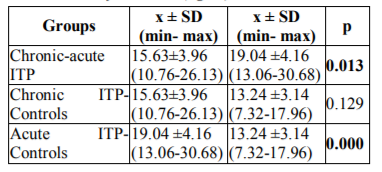
Serum 8-OHdG levels in patients with acute ITP were statistically significantly higher than in patients with chronic ITP and the controls (p=0.013 and p=0.0001, respectively). There was no statistically significant difference between serum 8-OHdG levels in patients with chronic ITP and the controls, although serum 8-OHdG level in patients with chronic ITP were higher than in the controls (p=0.129). Post-hoc pair wise comparisons between groups in terms of serum 8-OHdG levels are shown in Table3.
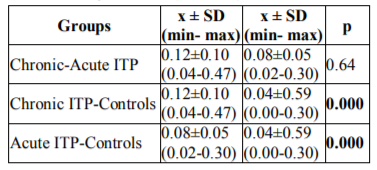
Statistically significant differences were observed between serum MDA levels in patients with chronic ITP and the controls, and in patients with acute ITP and the controls (p=0.0001 and p=0.0001, respectively). There was no statistically significant difference between serum MDA levels in patients with chronic and acute ITP (p=0.064). Post-hoc pair wise comparisons between the groups in terms of serum MDA levels are shown in Table 4.
There were no statistically significant differences between the serum protein carbonyl levels in the three groups (p=0.208). Post-hoc pair wise comparison analysis was therefore not performed.
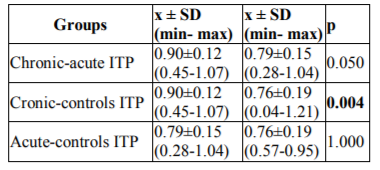
A statistically significant difference was determined between serum TAC levels in the patients with chronic ITP and the controls (p=0.004). There were no statistically significant differences between serum TAC levels in patients with chronic ITP and those with acute ITP, and in patients with acute ITP and the controls (p=0.05, and p=1.00, respectively). Post-hoc pair wise comparisons between the groups in terms of serum TAC levels are shown in Table 5.
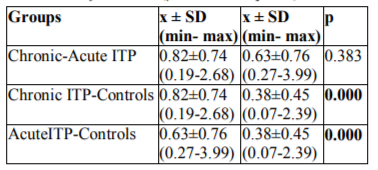
Serum TOS levels in patients with chronic and acute ITP were statistically significantly higher than in the controls (p= 0.0001, and p=0.0001, respectively). No significant difference was determined between serum TOS levels in patients with chronic and acute ITP (p=0.383). Post-hoc pair wise comparisons between the groups in terms of serum TOS levels are shown in Table 6.
No statistically significant differences were determined between serum OSI levels in the three groups (p=0.066). Post-hoc pair wise comparison analysis was therefore not performed. There were no correlation between platelet counts and serum TAS, TOS, OSI, MDA, protein carbonyl and 8-OHdG levels in patients with acute ITP, chronic ITP and controls (p>0.05).
5. Discussion
Despite continuing investigation, the pathogenesis of ITP remains unclear. The main hypotheses concerning the pathogenesis of ITP involve apoptosis, cytokine delivery, B- and T-lymphocyte dysfunction, macrophage Fc-gamma-IIA receptor polymorphism and oxidative injury. Increased oxidative injury contributes to inflammation and apoptosis, and lowers the functioning of the immune system [15]. Infection-related oxidative stress may induce a disturbed immune response. Antibodies or immune complexes against platelets lead to early destruction of platelets through phagocytosis or through cytotoxic T cells in predisposed individuals [16].
The most sensitive compounds to free radical injury are lipids. ROS cause peroxidation of cell membrane lipids and damage the integrity of the cell membrane. Membrane proteins, receptors and enzymes are inactivated as a result [17]. In this study, serum MDA levels in patients with acute and chronic ITP were higher than in the controls (p=0.0001 and p=0.0001, respectively). These findings indicate lipid peroxidation and lipid damage caused by ROS in both acute and chronic ITP.
Mean serum 8-OHdG levels in patients with acute ITP were higher than in patients with chronic ITP and higher than in the controls (p=0.013, and p=0.001, respectively). Mean serum 8-OHdG levels in patients with chronic ITP were higher than in the controls, although this was not statistically significant (p=0.129). These findings indicate that oxidation of DNA in particular occurs in patients with acute ITP, and that not all ROS cause oxidative injury to DNA because the DNA molecule is well preserved. The highest level of damage in DNA occurs due to hydroxyl radicals which are synthesized following oxidant exposure such as infection, as seen in patients with acute ITP since 80% of patients with acute ITP have a history of viral infections 3 weeks ago. [18]. These may explain why 8-OHdG levels were higher in patients with acute ITP than in patients with chronic ITP and higher than in the controls.
No statistically significant differences were observed between serum protein carbonyl levels in the three groups in this study (p>0.05). These results may indicate that ROS exhibit oxidative effects on DNA and lipids before proteins.
Serum TAC and TOS levels were higher in chronic ITP than in acute ITP (p=0.05, and p=0.383, respectively). Statistically significant differences were determined in serum TOS levels between patients with chronic ITP and the controls and also between patients with acute ITP and the controls (p=0.001, and p=0.001, respectively). Serum TAC levels were significantly higher in patients with chronic ITP than in the controls (p=0.004). Increased oxidative stress was detected in acute and chronic ITP. The response of TAC was inadequate in patients with acute ITP. In patients with chronic ITP, the increase in serum TAC levels was interpreted as a response to increased serum TOS levels. The absence of an increase in serum 8-OHdG levels may be attributed to DNA being protected against oxidative injury, because serum TAC levels did increase in patients with chronic ITP.
It is not clear whether oxidative injury is a cause or a consequence of the development of autoimmune diseases. An increase in oxidative stress has been determined in autoimmune diseases such as Henoch-Schonlein purpura, SLE, systemic sclerosis, rheumatoid arthritis (RA), hemolytic anemia, and type I diabetes mellitus [7,19]. Increased oxidative injury to proteins was observed in the studies concerned, although we observed no statistically significant increase in serum protein carbonyl in our study. Hassan et al. suggested that serum MDA levels increased and that serum levels and activity of glutathione decreased in patients with SLE and RA[19]. Polat et al. investigated lipid peroxidation and the role of antioxidants in adult patients with ITP. They reported higher serum levels of MDA and lower serum levels of ascorbic acid and glutathione in adult patients with ITP compared to controls [20]. Zhang et al. suggested that over expression of VNN1, an oxidative stress sensor in epithelial cells was most strongly associated with progression to chronic ITP and that exposure of blood mononuclear cells to oxidative stress inducers elicited dramatic up- regulation of VNN1. These results implicate oxidative stress pathways in the pathogenesis of chronic pediatric ITP [8,9]. Akbayram et al. suggested that the serum MDA, TOS and OSI levels were higher and serum TAC levels lower in acute and chronic ITP patients compared to controls [15]. Milz et al. observed a decrease in TAC and an increase in glutathione enzyme activity in patients with acute ITP [21]. We observed an increase in serum TOS and TAC levels in patients with both acute and chronic ITP in our study. The correlation were not found between platelet counts and serum TAS, TOS, OSI, MDA, protein carbonyl and 8-OHdG levels in patients with acute ITP, chronic ITP and controls. Its cause may be due to the lack of the number of the patients enrolled in this study The main negative aspect of this study is the small size of studied cohort since presence of the multi factors of the pathogenesis of ITP and oxidant- antioxidant status is not only one factor responsible from the pathogenesis of ITP. Other factors were not addressed in this study.
6. Conclusion
Serum 8-OHdG, MDA and TOS values increased in patients with acute and chronic ITP. However, serum levels of TAC increased only in patients with chronic ITP. These findings suggest that oxidative damage plays a role in the pathogenesis of acute and chronic ITP.
Acknowledgement
We thank to the Research Fund of Karadeniz Technical University for supporting the project.
References
- Rodeghiero F., Stasi R., Gernsheimer T., Michel M., Provan D, ArnoldD.M., Bussel J.B., CinesD.B., Chong B.H., Cooper N., Godeau ., LechnerK., MazzucconiM.G., McMillanR., Saz M.A., Imbach P., Blanchette V.,Kühne T., RuggeriM. and GeorgeJ.N., Standartization of terminology, d efinitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group, Blood. 113 (11), 2386 (2009). (Book style)
- JinC.Q., DongH.X., ChengP.P., ZhouJ.W., ZhengB.Y. and Liu F., Antioxidant status and oxidative stress in pateints with chronic ITP, Human Immunol. 77 (6), 482 (2013). (Book style)
- Zhou B.,Zhao H., Yang R.C., and Han Z.C., Multi-dysfunctional pathophysiology in ITP. Crit. Rev. Oncol/Hematol. 54(2): 107 (2005). (Book style)
- LiuX.G., LiJ.L., QinP.,RenJ. , Ma S.H., Sun L., Shi Y., Ji X.B., Zhu Y.Y., Ma D.X., Guo C.S., DuX., Hou M., Peng J., Determination of platelet-bound glycoprotein-specific autoanti bodies by flow cytometric immunobead assay in primary immune thrombocytopenia, Eur. J. Haematol. 86 (4), 339 (2011). (Book style)
- GernsheimerT.. Chronic idiopathic thrombo-cytopenic purpura: mechanisms of pathogenesis, Oncologist. 14 (1) 12 (2009). (Book style)
- GriffithsH.R.. ROS as signalling molecules in T cells--evidence for abnormal redox signalling in the autoimmune disease, rheumatoid arthritis, Redox Rep. 10 (6) 273 (2005). (Book style)
- FilippinL.I.,VercelinoR.,MarroniN.P., XavierR.M.. Redox signalling and the inflammatory response in rheumatoid arthritis. British Society for Immunology, Clin. Exp. Immunol. 152 (3) 415 (2008). (Book style)
- ZhangB., Zehnder J. L., Oxidative stress and immune thrombocytopenia,Semin. Hematol. 50 (3) 1 (2013).(Book style)
- Zhang B., LoC., Shen L., SoodR. , Jones C., Cusmano-Ozog K., Park-Snyder S.,Wong W., Jeng M., CowanT., Engleman E.G. , Zehnder J.L.,The role of vanin-1 and oxidative stress-related pathways in distinguishing acute and chronic pediatric ITP, Blood. 117 (11)4569 (2011). (Book style)
- P. Lanzkowsky, Manual of Pediatric Hematology and Oncology,5 th ed. Elsevier Inc, New York, 2011,pp. 321-345. (Book Chapter)
- ColeC.H., Rapid update on childhood immune thrombocytopenic purpura, J. Paediatr. Child Health. 48 (5) 378 (2012). (Book style)
- Erel O., A novel automated method to measure total antioxidant response against potent free radical reactions, Clin. Biochem. 37 (2) 112 (2004). (Book style)
- Erel O., A new automated colorimetric method for measuring total oxidant status, Clin. Biochem. 38 (12) 1103 (2005). (Book style)
- YagiK., Lipid peroxides and related radicals in clinical medicine, Adv. Exp. Med. Biol. 366 1-15 (1994). (Book style)
- AkbayramS., DoğanM., AkgünC.,MukulY., Peker E., BayA., CaksenH., Oner A.F. ,The association of Oxidant Status and Antioxidant Capacity in Children With Acute and Chronic ITP, J. Pediatr. Hematol. Oncol. 32 (4)277 (2010). (Book style)
- Imbach P. Oxidative stress may cause ITP, Blood. 117(17)4405 (2011).(Book style)
- ValkoM.,LeibfritzD.,MoncolJ., Cronin M.T., Mazur M., Telser J., Free radicals and antioxidants in normal phsiological functions and human disease, Int. J. Biochem. Cell. Biol. 39 (1) 44 (2007). (Book style)
- KohenR., Nyska A, Oxidation of biological systems: Oxidative stres phenomena, antioxidants, redox reactions, and methods for their quantification, Toxicol. Pathol. 30 (6) 620 (2002). (Book style)
- Hassan S.Z., Gheita T.A.,Kenawy S.A.,Fahim A.T.,El-Sorougy I.M.,IbdouM.S.,Oxidative stress in systemic lupus erythematosus and rheumatoid arthritis patients: relationship to disease manifestations and activity, Int. J. Rheum. Dis. 14 (4) 325 (2011). (Book style)
- Polat G.,Tamer L.,Tanriverdi K., Gürkan E., Baslamisli F., Atik U., Levels of malondialdehyde, glutathione and ascorbic acid in idiopathic thrombocytopenic purpura, East Afr. Med. J.79 (8) 446 (2002). (Book style)
- Kamhieh-MilzJ., BalG., Sterzer V., Kamhieh-MilzS., ArbachO., SalamaA., Reduced antioxidant capacities in platelets from patients with autoimmune thrombocytopenia purpura (ITP), Platelets 23(3)184 (2012). (Book style)