Information
Journal Policies
Advanced Corneal Imaging for Ectasia Diagnosis
Rui Carneiro-Freitas, MD1,2, Marcella Q. Salomão, MD2,3,4, Bernardo T. Lopes, MD2,3,4,Renato Ambrosio Jr, MD, PhD2,3,4*
2.Rio de Janeiro Corneal Tomography and Biomechanics Study Group.
3.Instituto de Olhos Renato Ambrósio, Rio de Janeiro, Brasil.
4.Department of Ophthalmology, Escola Paulista de Medicina- UNIFESP, São Paulo.
Copyright : © 2017 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The diagnosis of corneal ectasia has evolved tremendously over the last decades because of two plain reasons. First, progressive ectasia (keratectasia) arisen as a severe and intricate complication after common elective laser vison correction (LVC) procedures such as LASIK,[1] leading to the essential need to detecting mild ectasia cases or cases who present with high susceptibility for ectasia progression[2]. Second, the advent of alternative treatments for ectatic corneal diseases, such as corneal crosslinking, intracorneal ring segments (ICRS) and customized therapeutic ablations have brought up the inevitability for identifying the cases that will benefit from these procedures, and also for planning such procedures [3,4]
In 1938, Amsler pioneered the work for detecting mild forms of keratoconus, coining the term “forme fruste keratoconus” (FFKC) [5,6]. In longitudinal studies, Amsler used a Placido-disk Polaroid photokeratoscopy to describe irregular patterns on the keratoscopic reflex that did precede clinically detectable signs of keratoconus. The term “fruste” classically refers to an incomplete or abortive presentation of a disease, being also a condition that could progress or not, and opposite to the “forme plaine” or full blown form of the disease. However, FFKC is a complex term, for which there is no real consensus on the definition,4 so that mild or subclinical were advocated to be a more appropriate terminology. Besides nomen clature, Amsler’s method did not gain momentum due to its inherent complexity that made it hard to be reproduced by other researchers. In addition, the clinical relevance for detecting early ectatic disease was relatively limited at that time, which was correlated to the treatment options available for the disease.
In the early 1980s, when refractive surgery emerged as a new subspecialty, the need to comprise a detailed analysis of corneal shape for evaluating patients before and after such procedures become obvious. A quantitative analysis from the Corneascope was developed by Rowsey and coworkers,[7] in an approach that provided contour keratometry in all corneal meridians. Nevertheless, it was the introduction of computerized technologies for videokeratography what provided the first revolution in this field with the development of corneal topography [8]. Placido-disk based computerized corneal topography standardized a reproducible method, generating clinical data for interpretation [9]. Various topographical indexes, such as the ones described by Rabinowitz and McDonnell in 1989, were developed for detecting keratoconus [10]. Interestingly, such data allowed for identifying mild ectatic changes before the development of other clinical signs at slit lamp, or loosing distance spectacle corrected visual acuity (DCVA) [11,12]. Such ability has been the most important argument to support corneal topography as a mandatory exam to screen refractive candidates prior to LVC [13].
Nevertheless, the quest and the need to go beyond corneal topography became unmistakable with the increased number of cases that still developed ectasia despite of normal topography and no other apparent risk factors [14,15]. In addition, there are also cases with irregular corneas that would have been excluded from surgery based on anterior surface characteristics, but had uneventful LASIK with stable outcomes [16] Thereby, the need to augment both the sensitivity and specificity has become unquestionable when considering ectasia diagnosis and screening for ectasia risk prior to LVC.
The development of enhanced diagnostic capabilities should include two distinct elements. The conscious or rational use of objective data is fundamental, as subjective analysis of front surface curvature maps have significant variability among different experts in the field [17]. Also, the need to provide more detailed clinical data through advanced imaging techniques for characterizing the cornea beyond but not excluding front surface curvature data, including corneal tomography and bio mechanical assessments [18-20]
Corneal tomography depicts both the anterior and posterior corneal surfaces, also generating the pachymetric map of the whole cornea.18 This is possible using different technologies, including Scheimpflug imaging,[21,22] very high-frequency digital ultrasound (VHF-US),[23,24] and optical coherence tomography (OCT) [25,26] These methods have different characteristics but they all provide a vast amount of clinical data further than front surface curvature topographic characterization. Interestingly, it has been a challenge to demonstrate that such vast and complex data do provide an improvement on the accuracy to detect mild forms of ectasia over the well-established corneal topography method.2 While longitudinal analysis would provide the ideal design for such studies, the fellow eye with normal topography from patients with clinical ectasia detected in the ipsilateral eye have been considered a good alternative as these eyes have been referred to be forme fruste keratoconus by Klyce [27]. Interestingly, different Scheimpflug systems did involve such model of very asymmetric ectasia cases to develop and further demonstrate an improvement on sensitivity for detecting early forms of ectasia. These include studies involving the Orbscan,[28] Pentacam,[29] Sirius30 and Galilei.[31] These studies generate displays with indices derived from different methods for artificial intelligence to generate objective analysis from the complex tomographic data in order to facilitate clinical decision. For example, the Belin/Ambrósio Enhanced Ectasia Display (BAD), available in the Pentacam, combines the standard and enhanced BFS elevation maps of the front and back surfaces, and the thickness distribution data. Different tomographic parameters are presented as the standard deviation from normality towards disease (d values) and a final BAD-D parameter is calculated based on a regression analysis to maximize accuracy for detecting ectatic disease. Other displays are also available, such as the SCORE analyzer developed by Gatinel and Saad, which is available for the Orbscan [32].
Corneal imaging has evolved also to the ability to analyze individualized layers of the cornea such as the epithelium and Bowman’s layer. We refer to this method as segmental or layered corneal tomography. Reinstein and coworkers developed epithelium thickness indices for detecting keratoconus using VHF-US.[23,33] Huang and coworkers developed a similar approach using the OCT [25,26]. In addition, Sinha-Roy and coworkers analyzed the irregularity of the Bowman's layer (BL) in ectatic and normal corneas and developed a Bowman's roughness index (BRI) which improved, along with epithelial thickness data and BAD-D, the sensitivity for the detection of mild forms of ectasia in studies involving the fellow eye with normal topography from very asymmetric ectasia (VAE) cases.
Nevertheless, it is important to deliberate that some of these VAE cases may be factual unilateral ectasia cases [34] Curiously, there is a consensus that true unilateral keratoconus does not exist, but also that a secondary or induced ectasia, caused by a mechanical process, such as continuous eye rubbing, may occur unilaterally [4]
. This concept is in bargain with the two-hit hypothesis, which considers an underlying genetic predisposition along with external environmental factors [3]. An alternative study for assessing ectasia susceptibility involves the analysis of the preoperative state of cases that developed ectasia after LVC [35]. However, the available clinical data prior to surgery should be a limiting factor for the relevance of such studies. Different groups have designed risk assessment scores, such as the Ectasia Risk Score System (ERSS) by Randleman et al[36] and Ectasia Susceptibility Score (ESS) by Ambrósio et al.[35]
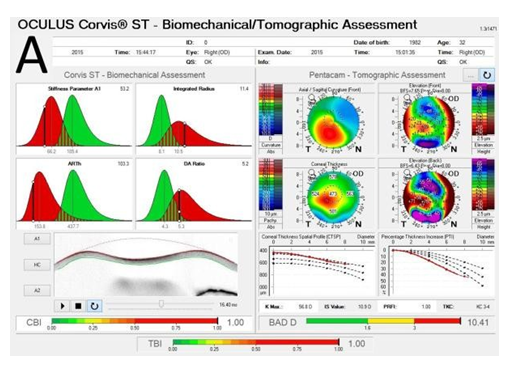
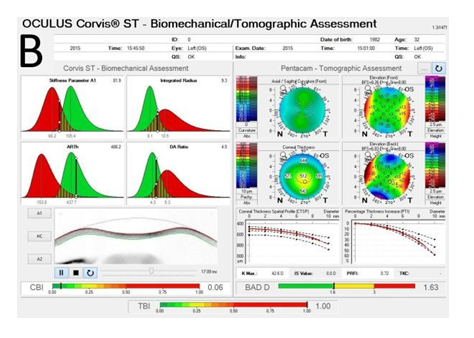
Recent advances in corneal biomechanics have contributed greatly for the understanding of ectasia pathophysiology.[37] The Ocular Response Analyzer® (Reichert Ophthalmic Instruments, Depew, NY)[38] and the Corvis® (OCULUS Optikgeräte GmbH; Wetzlar, Germany)[39] are non-contact t1onometers that monitor corneal deformation response. A novel biomechanical index, the CBI (Corneal Biomechanical Index) was developed by Vinciguerra and coworkers to integrate corneal deformation response (DCR) metrics from the Corvis ST.[40] Ultimately, the integration of biomechanical data and corneal shape data has been proposed for further improving accuracy to detect mild ectasia or even its susceptibility.[15,38] Recent studies were accomplished to develop the TBI (Tomographic Biomechanical Index), which combines from the Corvis ST and tomographic data from the Pentacam HR, through a random forest method with leave-one-out cross validation (RF-LOOCV). This novel index has shown very high accuracy for detecting ectasia, including a very high sensitivity for sub-clinical (fruste) ectasia among eyes with normal topography in very asymmetric patients, performing better than any other parameter tested (Figure 1) [41].
Future studies should consider the impact from LVC procedure and other factors from the environment, including as metrics that reflect ocular surface and allergy. These would be integrated to such shape and biomechanical parameters to assess the risk of ectasia progression. The prognostic information from these analysis would further improve our ability to better select refractive and therapeutic procedures.
References
- Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg 1998;24:1007-9.
- Ambrosio R, Jr., Randleman JB. Screening for ectasia risk: what are we screening for and how should we screen for it? J Refract Surg 2013; 29:230-2.
- McGhee CN, Kim BZ, Wilson PJ. Contemporary Treatment Paradigms in Keratoconus. Cornea 2015; 34 Suppl 10:S16-23.
- Gomes JA, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea 2015;34:359-69.
- Amsler M. Le kératocône fruste au Javal.Ophthalmologica 1938;96:77-83.
- Amsler M. [The "forme fruste" of keratoconus]. Wien Klin Wochenschr 1961;73:842-3.
- Rowsey JJ, Reynolds AE, Brown R. Corneal topography. Corneascope. Arch Ophthalmol 1981;99:1093-100.
- Wilson SE, Ambrosio R. Computerized corneal topography and its importance to wavefront technology. Cornea 2001;20:441-54.
- Klyce SD. Computer-assisted corneal topography. High-resolution graphic presentation and analysis of keratoscopy. Invest Ophthalmol Vis Sci 1984;25:1426-35.
- Rabinowitz YS, McDonnell PJ. Computer- assisted corneal topography in keratoconus. Refractive & corneal surgery 1989;5:400-8.
- Maeda N, Klyce SD, Smolek MK, Thompson HW. Automated keratoconus screening with corneal topography analysis. Investigative ophthalmology & visual science 1994;35:2749- 57.
- Maguire LJ, Bourne WM. Corneal topography of early keratoconus. American journal of ophthalmology 1989;108:107-12.
- Ambrosio R, Jr., Klyce SD, Wilson SE. Corneal topographic and pachymetric screening of keratorefractive patients. J Refract Surg 2003;19:24-9.
- Klein SR, Epstein RJ, Randleman JB, Stulting RD. Corneal ectasia after laser in situ keratomileusis in patients without apparent preoperative risk factors. Cornea 2006;25:388- 403.
- Ambrosio R, Jr., Dawson DG, Salomao M, Guerra FP, Caiado AL, Belin MW. Corneal ectasia after LASIK despite low preoperative risk: tomographic and biomechanical findings in the unoperated, stable, fellow eye. J Refract Surg 2010;26:906-11.
- Reinstein DZ, Archer TJ, Gobbe M. Stability of LASIK in topographically suspect keratoconus confirmed non-keratoconic by Artemis VHF digital ultrasound epithelial thickness mapping: 1-year follow-up. Journal of refractive surgery (Thorofare, NJ : 1995) 2009;25:569-77.
- Ramos IC, Correa R, Guerra FP, et al. Variability of subjective classifications of corneal topography maps from LASIK candidates. Journal of refractive surgery (Thorofare, NJ : 1995) 2013;29:770-5.
- Ambrosio R, Jr., Belin MW. Imaging of the cornea: topography vs tomography. J Refract Surg 2010;26:847-9.
- Ambrosio R, Jr., Nogueira LP, Caldas DL, et al. Evaluation of corneal shape and biomechanics before LASIK. International ophthalmology clinics 2011;51:11-38.
- Salomao MQ, Esposito A, Dupps WJ, Jr. Advances in anterior segment imaging and analysis. Curr Opin Ophthalmol 2009;20:324- 32.
- Ambrosio R, Jr., Valbon BF, Faria-Correia F, Ramos I, Luz A. Scheimpflug imaging for laser refractive surgery. Curr Opin Ophthalmol 2013;24:310-20.
- Belin MW, Ambrosio R. Scheimpflug imaging for keratoconus and ectatic disease. Indian J Ophthalmol 2013;61:401-6.
- Reinstein DZ, Archer TJ, Urs R, Gobbe M, RoyChoudhury A, Silverman RH. Detection of Keratoconus in Clinically and Algorithmically Topographically Normal Fellow Eyes Using Epithelial Thickness Analysis. J Refract Surg 2015;31:736-44.
- Reinstein DZ, Silverman RH, Rondeau MJ, Coleman DJ. Epithelial and corneal thickness measurements by high-frequency ultrasound digital signal processing. Ophthalmology 1994; 101:140-6.
- Li Y, Chamberlain W, Tan O, Brass R, Weiss JL, Huang D. Subclinical keratoconus detection by pattern analysis of corneal and epithelial thickness maps with optical coherence tomography. J Cataract Refract Surg 2016; 42:284-95.
- Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology 2012;119:2425-33.
- Klyce SD. Chasing the suspect: keratoconus. Br J Ophthalmol 2009;93:845-7.
- Saad A, Gatinel D. Topographic and tomo graphic properties of forme fruste keratoconus corneas. Investigative ophthalmology & visual science 2010;51:5546-55.
- Ambrosio R, Jr., Caiado AL, Guerra FP, et al. Novel pachymetric parameters based on corneal tomography for diagnosing keratoconus. Journal of refractive surgery (Thorofare, NJ : 1995) 2011;27:753-8.
- Arbelaez MC, Versaci F, Vestri G, Barboni P, Savini G. Use of a support vector machine for keratoconus and subclinical keratoconus detection by topographic and tomographic data. Ophthalmology 2012;119:2231-8.
- Smadja D, Touboul D, Cohen A, et al. Detection of subclinical keratoconus using an automated decision tree classification. American journal of ophthalmology 2013; 156:237-46.e1.
- Chan C, Ang M, Saad A, et al. Validation of an Objective Scoring System for Forme Fruste Keratoconus Detection and Post-LASIK Ectasia Risk Assessment in Asian Eyes. Cornea 2015;34:996-1004.
- Reinstein DZ, Gobbe M, Archer TJ, Silverman RH, Coleman DJ. Epithelial, stromal, and total corneal thickness in keratoconus: three- dimensional display with artemis very-high frequency digital ultrasound. J Refract Surg 2010;26:259-71.
- Isaac C Ramos DZR, Timothy J Archer, Marine Gobbe, Marcella Q Salomão, Bernardo Lopes, Allan Luz, Fernando Faria-Correia, Damien Gatinel, Michael W Belin, Renato Ambrósio Jr. Unilateral Ectasia characterized by Advanced Diagnostic Tests. International Journal of Keratoconus and Ectatic Corneal Diseases 2016;5:51.
- Ambrósio Jr R, Ramos I, Lopes B, et al. Assessing ectasia susceptibility prior to LASIK: the role of age and residual stromal bed (RSB) in conjunction to Belin-Ambrósio deviation index (BAD-D). Revista Brasileira de Oftalmologia 2014;73:75-80.
- Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology 2008;115:37-50.
- Roberts CJ, Dupps WJ, Jr. Biomechanics of corneal ectasia and biomechanical treatments. Journal of cataract and refractive surgery 2014;40:991-8.
- Luz A, Lopes B, Hallahan KM, et al. Enhanced Combined Tomography and Biomechanics Data for Distinguishing Forme Fruste Keratoconus. J Refract Surg 2016;32:479-94.
- Ambrósio Jr R, Ramos I, Luz A, et al. Dynamic ultra high speed Scheimpflug imaging for assessing corneal biomechanical properties. Revista Brasileira de Oftalmologia 2013;72:99- 102.
- Vinciguerra R, Ambrosio R, Jr., Elsheikh A, et al. Detection of Keratoconus With a New Biomechanical Index. J Refract Surg 2016; 32:803-10.
- Ambrosio R J, Lopes B., Faria-Correia F., Salomao M., Buhren J., Robets C., Elsheikh A., Vinciguerra R., Vinciguerra P. Integration of Scheimpflug based Corneal Tomography and Biomechanical Assessments for Enhancing Ectasia Detection. Journal of refractive surgery 2017.